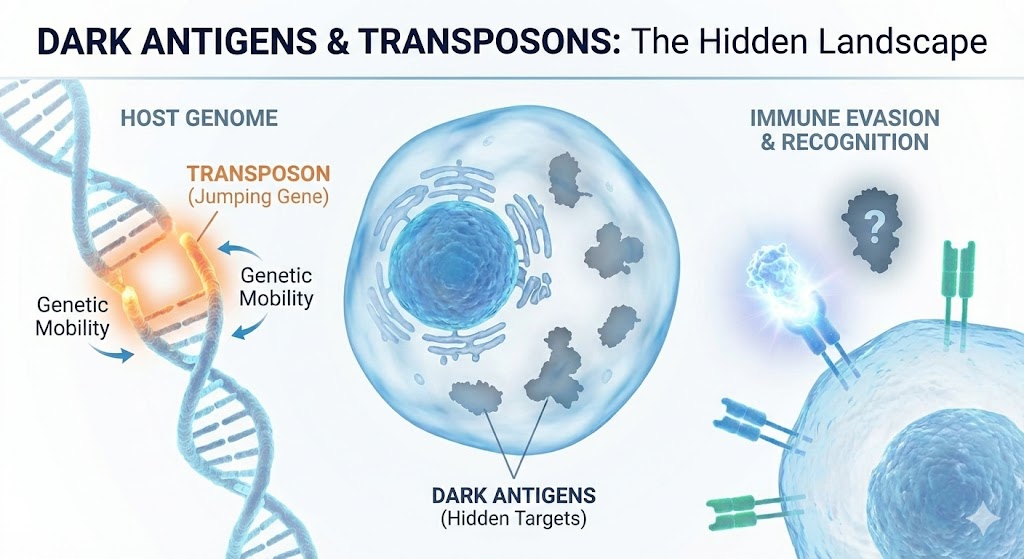
There are viral ghosts within us, lurking in our DNA. Transposons or Jumping genes are remnants of ancient retroviral infections. Almost half of our genome is made up of these viral DNA that has made its home in our Genome. They can form “dark antigens”- hidden viral proteins that evade immune detection and this biological invisibility drives conditions like cancer and autoimmunity.
If we could peel back the layers of the human genome and read it not as a database but as a biography, we would find that its earliest chapters were written not by cells but by viruses. Much of our DNA carries the ghostly signatures of ancient infections- fragments of prehistoric retroviruses, broken viral enzymes, scattered genetic husks that once replicated with ruthless efficiency.
Transposons “Jumping genes” are remnants of ancient viruses.
These viral traces- some stretching back tens of millions of years- did not simply infect our ancestors and vanish. Instead, they slipped into eggs or sperm and became permanent passengers in our lineage. Their structures decayed over time, their infectious abilities fell away, yet their DNA remained, tucked into our genome like forgotten footnotes left by civilizations long gone. These remnants, known as transposons or “jumping genes,” no longer resemble the viruses they once were, but they bear the unmistakable signature of an outsider.
The tale begins in deep evolutionary time. A virus infected a distant ancestor and quietly inserted its genetic material into a reproductive cell. Instead of dying with that host, the viral DNA became part of the heritable lineage. Over millions of years, such accidents accumulated. The machinery that once hijacked cells fell apart, leaving only genetic fossils behind- silent markers of ancient invasions. These transposons, these drifting genomic relics, became the heirs of those primeval encounters. Today most lie inactive, but nearly half of the human genome is composed of such fragments.
These fragments do far more than linger, they shape us in ways both subtle and profound. Some have been repurposed by evolution itself. A viral-derived gene helps form the human placenta. Other sequences are activated only in the earliest stages of embryonic development, orchestrating patterns of gene expression essential for life. Still others sleep quietly, surfacing only under stress or disease. Their presence reveals an unsettling truth: the human genome is not a pristine blueprint authored solely by our species. It is a palimpsest- written and rewritten across eras- layered with the scribbles and scars of viral encounters.
Dark antigens- Hidden triggers inside our body
This coexistence with ancient infections gives rise to a deeper biological mystery: how does the immune system interpret such a landscape? The answer is that it often does not. The immune system surveys only a curated selection of the body’s molecular contents. Many proteins, peptides, and cellular fragments are never displayed to immune cells at all. They exist behind a kind of biological veil. These rarely seen molecules are what scientists call dark antigens- elements present within us but almost entirely hidden from immune recognition.
This concept is more than a curiosity. Dark antigens emerge because not all cellular proteins are fed into the machinery that displays fragments to T cells. Some proteins exist only in specialized tissues the immune system rarely patrols. Others fold or degrade so quickly that no peptide fragments reach the antigen-presentation pathway. Some appear only in early development, long before the immune system has matured enough to classify them as self. And some come from transposons: fleeting proteins that appear briefly, vanish quickly, and leave no imprint on immune memory.
Viruses and their shadow biology to stay hidden.
The idea becomes even more intriguing when we realize it applies not only to fragments of our own genome but also to active viruses infecting humans today. Many viruses have evolved to keep portions of themselves hidden within this same darkness. They commandeer cellular machinery, block antigen processing, or cause infected cells to downregulate the molecules responsible for presenting viral fragments on the cell surface. Cytomegalovirus (CMV) retains MHC molecules inside the cell. Adenovirus (hAdV) traps the MHC complex in the endoplasmic reticulum. HIV removes these molecules from the cell surface entirely through its Nef protein.
By doing so, viruses generate a shadow-space within the cell- an internal landscape where viral proteins exist but remain unseen. They create their own dark antigens, viral shapes that exist within us but remain invisible to the immune defenses meant to recognize them.
Members of families like Herpesviridae (HSV, EBV)- which include the agents responsible for cold sores, chickenpox, and mononucleosis- take this strategy even further. They enter a latent state inside our cells and produce almost no detectable proteins. They survive not by fighting the immune system but by embracing molecular silence. Retroviruses such as HIV employ a different form of invisibility, integrating into the host genome and selectively expressing proteins that evade immune attacks.
Others such as Hepatitis B virus partially escape detection by continuously reshaping the antigen landscape. HPV persists in the epithelial layers where immune surveillance is naturally sparse. Meanwhile, most retroviruses that once infected our ancestors have been inherited in the germline and now exist as transposons- dormant, fragmented, but still capable of awakening. These modern pathogens and our ancient viral fossils converge on the same biological principle: survival often depends on what remains unseen.
Dark antigens as culprits of Cancer and other Neurodegenerative diseases
The consequences of such invisibility stretch far beyond infection. Some viruses do more than hide; they fundamentally reprogram the cells they infect. This is clearest in viruses that cause cancer. HPV, for example, produces the E6 and E7 proteins that disable the cell’s tumor-suppressor genes, clearing the way for uncontrolled growth. Epstein-Barr virus drives B cells into states of perpetual activation, paving the way for lymphomas. Hepatitis B virus inflames liver tissue over decades, accelerating DNA damage until cancer emerges.
Cancer, in these contexts, becomes the unintended byproduct of viral persistence- the molecular cost of survival strategies that reshape the very cells the virus inhabits.
Autoimmune disease tells a similar story of unintended consequences. When the immune system clears a viral infection, it expands armies of T cells and antibodies. But some viral proteins closely resemble human ones. This phenomenon- molecular mimicry- can cause the immune system to mistake the body’s tissues for lingering viral debris. Infections with enteroviruses such as Coxsackievirus B have long been linked to autoimmune diabetes. Epstein-Barr virus contains sequences resembling myelin proteins, offering one explanation for its association with multiple sclerosis.
Dark antigens magnify this risk. When a tissue normally hidden from immune view is injured, its antigens spill into circulation for the first time. An immune system primed by infection may misinterpret these newly revealed fragments as viral mimics- and attack. In multiple neurological disorders, fragments of transposon-derived RNA have been detected in inflamed brain regions, raising questions about whether ancient viral remnants can influence conditions such as multiple sclerosis.
In cancer immunotherapy, dark antigens have become a source of hope. Tumors often loosen the tightly wound DNA structures that normally silence ancient viral fragments. Endogenous retroviruses, once entombed in the genome, awaken in these cancer cells. Their reactivated proteins resemble those of ancient pathogens the immune system has never encountered. Some of the success of checkpoint inhibitors may lie in unmasking these neo-expressed viral fossils. Yet tumors also exploit this same mechanism, using ERV activation to create confusing RNA patterns that mimic viral infection and generate inflammation that shields the tumor.
Chronic viral infections reshape immunity even more broadly. Herpesviruses, HIV, and hepatitis C remain inside the body for decades, gradually altering the architecture of immune memory. Cytomegalovirus can occupy a substantial portion of an older adult’s T-cell repertoire; HIV dismantles entire immune cell subsets; hepatitis C remodels the liver so thoroughly that immune surveillance changes permanently.
These shifts influence not only how we respond to pathogens but how we respond to ourselves.
Interplay between Dark antigens and Immune surveillance.
This interplay between darkness and recognition shapes evolution as well. Carrying viral remnants forces the immune system to evolve tolerance. Viruses that evolve new forms of invisibility compel hosts to develop new kinds of detection. This reciprocal shaping has sculpted the interferon system, driven the extraordinary diversity of the MHC region, and embedded viral regulatory sequences into the very logic of our gene networks. What once were invasions have become regulatory architecture.
To understand ourselves, then, is to acknowledge that we are composite beings. We carry human genes, yes, but also layers of viral history and immune interpretation. The immune system, for all its perceptive power, navigates a world where much of what matters is only dimly visible. Its successes, its failures, and its occasional miscalculations all reflect the complexity of distinguishing self from stranger in a genome that is neither wholly one nor the other.
The story of dark antigens and viral remnants is, in the end, a story about perspective- what the immune system sees, what it overlooks, and what it cannot yet understand. It reminds us that biology is not simply a contest of strength but a negotiation of visibility. The most enduring entities are not always the most aggressive but often the most silent. They persist not by overpowering the host but by becoming part of its fabric, shaping its evolution and its vulnerabilities from within.

Leave a comment